
pharmacovigilance

Clinical Trial Services

Pre Clinical Services
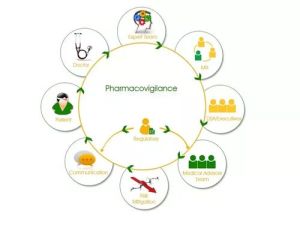
Pharmacovigilance Services
pharmacovigilance system establishment development of risk management plan (rmp), periodic safety update reports (psur)pbrerpader safety data exchange agreements safety database technical team trainings icsrs: processing & reporting qppv services audit & inspection support signal detection & management global & local literature surveillance xevmpdidmp support other pharmacovigilance consulting support.
...more
intellectual property right services
Intellectual property evaluation of product process patents development strategies as per non infringing technologies patent drafting and submission.
...more
Formulation Development Service
During stages of early product development, medwisdom can offer useful advice on issues of importance for future expedient approval. In our specialised field of pharmaceuticals, biologics, biotech, cosmetics, herbal, medical device. We inform clients of requirements for manufacturing and control, emphasising the importance of the documenting process as well as product consistency. We have expertise in the design of assays, and can develop statistical models for their evaluation according to pharmacopoeial requirements. Review of production processes and facilities and design of validation protocols are also within our capabilities. In addition, we can produce qualified input for the planning of appropriate stability studies, non-clinical studies and clinical trials.
...more
dossier services
Dossiers (ctd) compilation for submissions in: asia africa middle east central america south america north america dossier compilation in common technical dossier (ctd) format and conversion to ectd for: us fda saudi fda european counties dmf (drug master file) and cep.
...more
Auditing Services
Medwisdom is regularly involved in auditing or issues related to gxp. We are called upon by clients to conduct gmp inspections or provide technicaldocumentation reviews with respect to gmp issues, either as part of a larger strategic review or as due diligence for selection of third party contractors. We can offer the following services in this area: gmp medwisdom contributes to the auditing process by documentation review to ensure that product-specific issues are addressed, such as requirements in shared manufacturing facilities. Our expertise has also been called upon during gmp inspections, either to aid in the inspection process or to liase with the inspecting authorities. Qms perform pre-audit for qms (iso 9001 and iso 13485)
...more
dossier services

pharmacovigilance

intellectual property right services

Formulation Development Service

Clinical Trial Services

Pre Clinical Services
Be first to Rate
Rate ThisOpening Hours